Multiple Choice
Determine the change in enthalpy for the following reaction: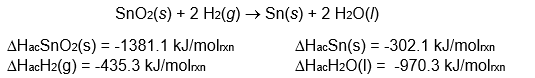
A) -3088 kJ/molrxn
B) +544 kJ/molrxn
C) -544 kJ/molrxn
D) +9.0 kJ/molrxn
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Use the following standard enthalpies of
Q21: (Note that some of these
Q22: The disposable lighters that many smokers carry
Q23: Calculate <span class="ql-formula" data-value="\Delta"><span
Q24: (Note that some of these
Q26: The following questions often assume that a
Q27: Consider the following data for heats of
Q28: The following questions often assume that a
Q29: (Note that some of these
Q30: Use your understanding of the bonding