Multiple Choice
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-What is the sign of the enthalpy of reaction for the reaction P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?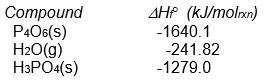
A) positive
B) negative
C) impossible to determine from the data
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Which of the following is an
Q20: Use the following standard enthalpies of
Q21: (Note that some of these
Q22: The disposable lighters that many smokers carry
Q23: Calculate <span class="ql-formula" data-value="\Delta"><span
Q25: Determine the change in enthalpy for the
Q26: The following questions often assume that a
Q27: Consider the following data for heats of
Q28: The following questions often assume that a
Q29: (Note that some of these