Multiple Choice
Calculate Hrxn° for the reaction: 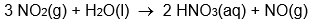
from the following enthalpy of atom combination data.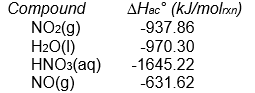
A) less than -1000 kJ/molrxn
B) between -1000 and -750 kJ/molrxn
C) between -750 and -500 kJ/molrxn
D) between -500 and -250 kJ/molrxn
E) between -250 and 0 kJ/molrxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: For the reaction<br>MnO<sub>2</sub>(s) + CO(g)
Q19: Which of the following is an
Q20: Use the following standard enthalpies of
Q21: (Note that some of these
Q22: The disposable lighters that many smokers carry
Q24: (Note that some of these
Q25: Determine the change in enthalpy for the
Q26: The following questions often assume that a
Q27: Consider the following data for heats of
Q28: The following questions often assume that a