Essay
The disposable lighters that many smokers carry use butane as a fuel. Butane occurs as two isomers, n-butane 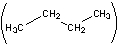
and isobutane
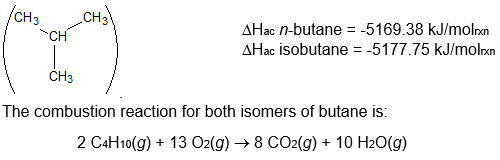
(I) Which form of butane will release the most heat when it is burned by the above combustion reaction? Explain your reasoning.
(II) Which form of butane will have the strongest bonds? Explain your reasoning.
Correct Answer:

Verified
(I) Both forms of butane produce the exa...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: The bond strength between C and a
Q18: For the reaction<br>MnO<sub>2</sub>(s) + CO(g)
Q19: Which of the following is an
Q20: Use the following standard enthalpies of
Q21: (Note that some of these
Q23: Calculate <span class="ql-formula" data-value="\Delta"><span
Q24: (Note that some of these
Q25: Determine the change in enthalpy for the
Q26: The following questions often assume that a
Q27: Consider the following data for heats of