Essay
Use enthalpies of atom combination to calculate Hrxn° for the following reaction.
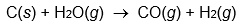
What would happen to the magnitude of Hrxn° if the reaction consumed liquid water instead of gaseous water?

Correct Answer:

Verified
132 kJ/molrxnView Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q34: The enthalpy of atom combination for CCl<sub>4</sub>(g)
Q35: Arrange the following in order of increasing
Q36: How much heat in kilojoules is
Q37: (Note that some of these are
Q38: Both ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) and methanol (CH<sub>3</sub>OH) have
Q40: How much heat is produced when 0.200
Q41: Which of the following reactions is
Q42: (Note that some of these
Q43: The following questions often assume that a
Q44: (Note that some of these