Multiple Choice
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-How much energy in joules is required to heat 10.0 grams of gold from room temperature (20.0°C) to the temperature of boiling water (100.0°C) if the specific heat of gold is 0.129 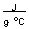 ?
?
A) 1.29 J
B) 10.3 J
C) 103 J
D) 6,200 J
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q38: Both ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) and methanol (CH<sub>3</sub>OH) have
Q39: Use enthalpies of atom combination to
Q40: How much heat is produced when 0.200
Q41: Which of the following reactions is
Q42: (Note that some of these
Q44: (Note that some of these
Q45: Calculate <span class="ql-formula" data-value="\Delta"><span
Q46: Calculate <span class="ql-formula" data-value="\Delta"><span
Q47: Use enthalpies of atom combination to calculate
Q48: (Note that some of these