Short Answer
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Given that H ° for this reaction is -5645 kJ/molrxn, what is the value of Hf ° for sucrose?
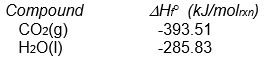
Correct Answer:

Verified
-2221 kJ/m...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q37: (Note that some of these are
Q38: Both ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) and methanol (CH<sub>3</sub>OH) have
Q39: Use enthalpies of atom combination to
Q40: How much heat is produced when 0.200
Q41: Which of the following reactions is
Q43: The following questions often assume that a
Q44: (Note that some of these
Q45: Calculate <span class="ql-formula" data-value="\Delta"><span
Q46: Calculate <span class="ql-formula" data-value="\Delta"><span
Q47: Use enthalpies of atom combination to calculate