Multiple Choice
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Calculate H ° for the following reaction from the data given below.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
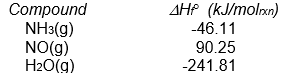
A) -105.5 kJ/molrxn
B) -905.4 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Use enthalpies of atom combination to
Q40: How much heat is produced when 0.200
Q41: Which of the following reactions is
Q42: (Note that some of these
Q43: The following questions often assume that a
Q45: Calculate <span class="ql-formula" data-value="\Delta"><span
Q46: Calculate <span class="ql-formula" data-value="\Delta"><span
Q47: Use enthalpies of atom combination to calculate
Q48: (Note that some of these
Q49: (Note that some of these