Multiple Choice
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules.Which reaction mixture is at equilibrium? 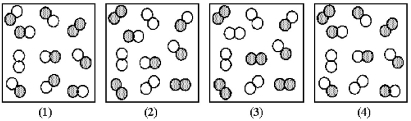
A) reaction mixture (1)
B) reaction mixture (2)
C) reaction mixture (3)
D) reaction mixture (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q50: Nitric oxide reacts with oxygen to form
Q62: For which one of the following reactions
Q63: Shown below is a concentration vs.time plot
Q66: What is the value for K<sub>c</sub> for
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q135: Picture (1)represents an equilibrium mixture of solid
Q155: Picture (1)represents an equilibrium mixture of solid
Q161: Acids donate protons to water according to