Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 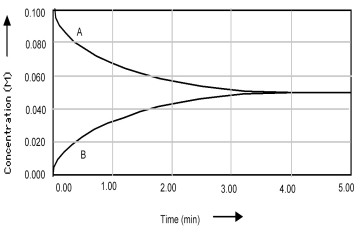
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: Nitric oxide reacts with oxygen to form
Q58: What is the equilibrium equation for the
Q62: For which one of the following reactions
Q64: The reaction A<sub>2</sub> + B<sub>2</sub> ⇌ 2
Q66: What is the value for K<sub>c</sub> for
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q135: Picture (1)represents an equilibrium mixture of solid
Q155: Picture (1)represents an equilibrium mixture of solid
Q161: Acids donate protons to water according to