Multiple Choice
For the reaction HCONH2(g) 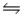 NH3(g) + CO(g) , Kc = 4.84 at 400 K.If H° for this reaction is 29 kJ/mol, find Kc at 500 K.
NH3(g) + CO(g) , Kc = 4.84 at 400 K.If H° for this reaction is 29 kJ/mol, find Kc at 500 K.
A) 5.8
B) 0.17
C) 27
D) 0.88
E) 10.3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: A sample of solid naphthalene is introduced
Q26: Given the following data, estimate the
Q27: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q30: Find the temperature at which K<sub>p</sub>
Q32: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q33: The equilibrium constant for the reaction
Q38: The entropy of any pure substance at
Q61: For a given substance the entropy always
Q64: Melting an ionic solid always results in
Q109: Without reference to a table, arrange