Short Answer
For the reaction SbCl5(g) 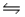
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 800 K and 1 atm pressure.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q28: For the reaction HCONH<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q30: Find the temperature at which K<sub>p</sub>
Q33: The equilibrium constant for the reaction
Q34: Sulfur can be separated from lead
Q35: Under what conditions (always, never, high
Q36: For the reaction H<sub>2</sub>O<sub>2</sub>(g) <span class="ql-formula"
Q37: The reaction rates of many spontaneous
Q64: Melting an ionic solid always results in
Q109: Without reference to a table, arrange