Short Answer
2.0 L of a ideal nitrogen gas (N2)are at 0.00°C and 1.0 atm.The ideal gas constant is 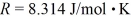 = 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
= 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
(a)Determine the number of moles of N2.
(b)How many molecules of N2 are present?
(c)What is the mass of this gas?
Correct Answer:

Verified
(a)0.089 m...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: The interior of a refrigerator has a
Q11: Which contains more moles of material: 80
Q12: For a fixed amount of gas,if the
Q13: The root-mean-square speed (thermal speed)for a certain
Q14: The mean free path of an oxygen
Q16: A bag of potato chips contains 2.00
Q17: What is the mean free path of
Q18: A 3.2-L volume of neon gas (Ne)is
Q19: 3.00 moles of an ideal gas at
Q20: A cubic box with sides of 20.0