Multiple Choice
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed) of 200 m/s.The mass of a helium molecule is 3.40 × 10-27 kg.What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is 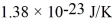 and the ideal gas constant is
and the ideal gas constant is 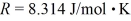 =
= 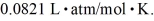
A) 3.39 kPa
B) 1.13 kPa
C) 570 Pa
D) 2.26 kPa
E) 9.10 Pa
Correct Answer:

Verified
Correct Answer:
Verified
Q15: 2.0 L of a ideal nitrogen gas
Q16: A bag of potato chips contains 2.00
Q17: What is the mean free path of
Q18: A 3.2-L volume of neon gas (Ne)is
Q19: 3.00 moles of an ideal gas at
Q21: An ideal gas is kept in a
Q22: A fixed amount of ideal gas is
Q23: The mean free path of an oxygen
Q24: What is the mass density of argon
Q25: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed