Multiple Choice
What is the mean free path of molecules in an ideal gas in which the mean collision time is 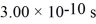 ,the temperature is 300 K,and the mass of the molecules is
,the temperature is 300 K,and the mass of the molecules is 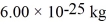 ? Assume that the molecules are moving at their root-mean-square speeds.The Boltzmann constant is
? Assume that the molecules are moving at their root-mean-square speeds.The Boltzmann constant is 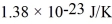 .
.
A) 7.22 × 10-8 m
B) 4.32 × 10-8 m
C) 9.19 × 10-8 m
D) 1.39 × 10-8 m
E) 6.71 × 10-8 m
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: For a fixed amount of gas,if the
Q13: The root-mean-square speed (thermal speed)for a certain
Q14: The mean free path of an oxygen
Q15: 2.0 L of a ideal nitrogen gas
Q16: A bag of potato chips contains 2.00
Q18: A 3.2-L volume of neon gas (Ne)is
Q19: 3.00 moles of an ideal gas at
Q20: A cubic box with sides of 20.0
Q21: An ideal gas is kept in a
Q22: A fixed amount of ideal gas is