Multiple Choice
3.00 moles of an ideal gas at a pressure of 250 kPa are held in a container of volume of 25.0 L.The ideal gas constant is R = 8.314 J/mol • K = 0.0821 L ∙ atm/mol • K,and 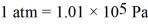 .The temperature of this gas is closest to
.The temperature of this gas is closest to
A) 240°C.
B) -180°C.
C) 480°C.
D) -1.0°C.
E) -22°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The mean free path of an oxygen
Q15: 2.0 L of a ideal nitrogen gas
Q16: A bag of potato chips contains 2.00
Q17: What is the mean free path of
Q18: A 3.2-L volume of neon gas (Ne)is
Q20: A cubic box with sides of 20.0
Q21: An ideal gas is kept in a
Q22: A fixed amount of ideal gas is
Q23: The mean free path of an oxygen
Q24: What is the mass density of argon