Multiple Choice
How many moles of water (H2O) molecules are in a 4.00 m3 container at a pressure 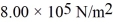 and temperature 600°C? The ideal gas constant is
and temperature 600°C? The ideal gas constant is 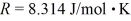 =
= 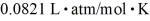 .
.
A) 7.72 × 1026 mol
B) 641 mol
C) 441 mol
D) 3.86 × 1026 mol
E) 2.65 × 1026 mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: An ideal gas is kept in a
Q4: An ideal gas is at a pressure
Q5: What is the total translational kinetic energy
Q6: What is the mean free path for
Q7: An oxygen molecule falls in a vacuum.From
Q9: On a hot summer day,the temperature is
Q10: The interior of a refrigerator has a
Q11: Which contains more moles of material: 80
Q12: For a fixed amount of gas,if the
Q13: The root-mean-square speed (thermal speed)for a certain