Short Answer
On a hot summer day,the temperature is 40.0°C and the pressure is 1.01 × 105 Pa.Let us model the air as all nitrogen of molecular mass 28.0 g/mol having molecules of diameter 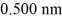 that are moving at their root-mean-square speed.Avogadro's number is
that are moving at their root-mean-square speed.Avogadro's number is 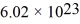 molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is
molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is 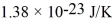 .Calculate reasonable estimates for
.Calculate reasonable estimates for
(a)the root-mean-square speed of the nitrogen molecules.
(b)the average distance a typical molecule travels between collisions.
(c)the average time a molecule travels between collisions,assuming that the molecules are moving at their root-mean-square speeds.
(d)the number of collisions an average molecule undergoes per second.
Correct Answer:

Verified
(a)528 m/s
(b)3.85 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(b)3.85 ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q4: An ideal gas is at a pressure
Q5: What is the total translational kinetic energy
Q6: What is the mean free path for
Q7: An oxygen molecule falls in a vacuum.From
Q8: How many moles of water (H<sub>2</sub>O)molecules are
Q10: The interior of a refrigerator has a
Q11: Which contains more moles of material: 80
Q12: For a fixed amount of gas,if the
Q13: The root-mean-square speed (thermal speed)for a certain
Q14: The mean free path of an oxygen