Multiple Choice
An oxygen molecule falls in a vacuum.From what height must it fall so that its kinetic energy at the bottom equals the average energy of an oxygen molecule at 800 K? (The Boltzmann constant is 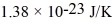 ,the molecular weight of oxygen is 32.0 g/mol,and Avogadro's number is
,the molecular weight of oxygen is 32.0 g/mol,and Avogadro's number is 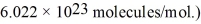
A) 31.8 km
B) 10.6 km
C) 21.1 km
D) 42.3 km
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: If a certain sample of an ideal
Q3: An ideal gas is kept in a
Q4: An ideal gas is at a pressure
Q5: What is the total translational kinetic energy
Q6: What is the mean free path for
Q8: How many moles of water (H<sub>2</sub>O)molecules are
Q9: On a hot summer day,the temperature is
Q10: The interior of a refrigerator has a
Q11: Which contains more moles of material: 80
Q12: For a fixed amount of gas,if the