Multiple Choice
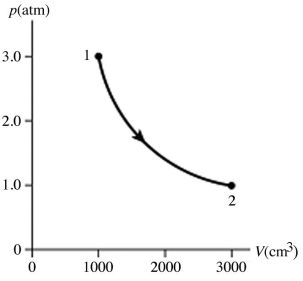
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2.The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1.What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol • K = 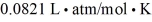 .
. 
A) -160°C
B) 15°C
C) 390°C
D) 120°C
Correct Answer:

Verified
Correct Answer:
Verified
Q17: A monatomic ideal gas undergoes an isothermal
Q18: An ideal gas increases in temperature from
Q19: 3.0 moles of an ideal gas with
Q20: A cylinder contains 1.2 moles of ideal
Q21: A cylinder contains 24.0 moles of an
Q23: The process shown in the pV diagram
Q24: When an ideal gas increases in volume
Q25: The figure (not to scale)shows a pV
Q26: An ideal gas is allowed to expand
Q27: A container with rigid walls is filled