Multiple Choice
Which of the following is always true for a reaction where Kc is 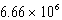 at 25 C?
at 25 C?
A) The reaction mixture contains mostly products at equilibrium.
B) The reaction mixture contains mostly reactants at equilibrium.
C) The rate of reaction is very fast.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The symbol Q is called the _.
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q47: The reaction quotient,Q,for a system is <img
Q48: What is the K<sub>c</sub> expression for the
Q49: For the reaction NO(g)+ ½ O<sub>2</sub>(g)
Q52: For which of the following reactions are
Q53: For the equilibrium N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q54: Write the expression for K for the
Q55: Assume that the following <span
Q56: A mixture of nitrogen and hydrogen was