Multiple Choice
The reaction quotient,Q,for a system is 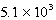 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 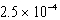
,what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net gain in both product(s) and reactant(s) .
C) There will be a net gain in product(s) .
D) There will be a net gain in reactant(s) .
E) The equilibrium constant will decrease until it equals the reaction quotient.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The symbol Q is called the _.
Q42: Assume that the following chemical reaction
Q43: What is the reaction quotient,Q,for the equilibrium<br><img
Q44: When gaseous carbon monoxide and hydrogen are
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q48: What is the K<sub>c</sub> expression for the
Q49: For the reaction NO(g)+ ½ O<sub>2</sub>(g)
Q51: Which of the following is always
Q52: For which of the following reactions are