Multiple Choice
At 25 C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below?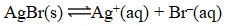
A) 5.40 10-13
B) 5.40 10-11
C) 1.90 10-8
D) 7.35 10-7
E) 1.90 10-6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: The symbol Q is called the _.
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q42: Assume that the following chemical reaction
Q43: What is the reaction quotient,Q,for the equilibrium<br><img
Q44: When gaseous carbon monoxide and hydrogen are
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q47: The reaction quotient,Q,for a system is <img
Q48: What is the K<sub>c</sub> expression for the
Q49: For the reaction NO(g)+ ½ O<sub>2</sub>(g)
Q51: Which of the following is always