Multiple Choice
What is the reaction quotient,Q,for the equilibrium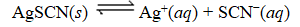 SCN-(aq)
SCN-(aq)
When 0.4257 L of 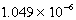
M Ag+ is combined with 0.2376 L of 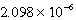
M SCN- in the presence of an excess of AgSCN(s) ?
A) 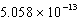
B) 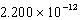
C) 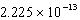
D) 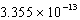
E) 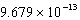
Correct Answer:

Verified
Correct Answer:
Verified
Q38: A flask contains the following chemical system
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q42: Assume that the following chemical reaction
Q44: When gaseous carbon monoxide and hydrogen are
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q47: The reaction quotient,Q,for a system is <img
Q48: What is the K<sub>c</sub> expression for the