Multiple Choice
A 50.0-mL sample of 0.100 M Ca(NO3) 2 is mixed with 50.00 mL of 0.200 M NaF.When the system has come to equilibrium,which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 10-11. Moles Solid CaF2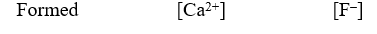
A) 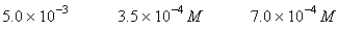
B) 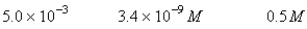
C) 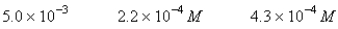
D) 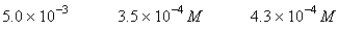
E) 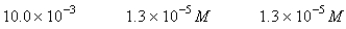
Correct Answer:

Verified
Correct Answer:
Verified
Q52: The solubility of an unknown salt,M<sub>3</sub>Z<sub>2,</sub> at
Q53: The solubility of AgCl in water is
Q54: The solubility of Cd(OH)<sub>2</sub> in water
Q55: A 100.-mL sample of solution contains
Q56: The concentration of Mg<sup>2+</sup> in seawater
Q58: The solubility of an unknown salt,MZ<sub>2,</sub> at
Q59: The K<sub>sp</sub> of an unknown salt,MZ<sub>2</sub>,is <img
Q60: Which of the following compounds has
Q61: You have two salts,AgX and AgY,with very
Q62: What is the molar solubility of