Multiple Choice
The solubility of an unknown salt,MZ2, at 25°C is 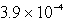 mol/L.What is the Ksp for MZ2 at 25°C?
mol/L.What is the Ksp for MZ2 at 25°C?
A) 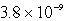
B) 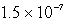
C) 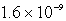
D) 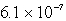
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: The solubility of AgCl in water is
Q54: The solubility of Cd(OH)<sub>2</sub> in water
Q55: A 100.-mL sample of solution contains
Q56: The concentration of Mg<sup>2+</sup> in seawater
Q57: A 50.0-mL sample of 0.100 M
Q59: The K<sub>sp</sub> of an unknown salt,MZ<sub>2</sub>,is <img
Q60: Which of the following compounds has
Q61: You have two salts,AgX and AgY,with very
Q62: What is the molar solubility of
Q63: The K<sub>sp</sub> for BaF<sub>2</sub> is 2.4