Multiple Choice
Consider the following rate law: 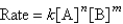 How are the exponents n and m determined?
How are the exponents n and m determined?
A) by using the balanced chemical equation
B) by using the subscripts for the chemical formulas
C) by using the coefficients of the chemical formulas
D) by educated guess
E) by experiment
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Two mechanisms are proposed: I. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q64: What is the numerical value of the
Q65: For the second-order reaction NO(g)+ O<sub>3</sub>(g)
Q66: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="For the
Q67: The rate is constant over time.
Q69: The elementary chemical reaction O +
Q70: The rate constant k is dependent on<br>I.the
Q71: Consider the reaction 3A + B
Q72: The reaction H<sub>2</sub>SeO<sub>3</sub>(aq)6I<sup>-</sup>(aq)+ 4H<sup>+</sup>(aq) <span class="ql-formula"
Q73: If the reaction were reversible,would the forward