Multiple Choice
Two mechanisms are proposed: I. 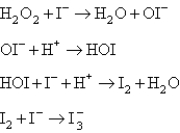 II.
II. 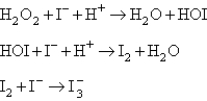 Which mechanism and which step as the rate determining step would best fit the data?
Which mechanism and which step as the rate determining step would best fit the data?
A) Mechanism I,with the first step the rate determining step.
B) Mechanism I,with the second step the rate determining step.
C) Mechanism II,with the first step rate determining.
D) Mechanism II,with the second step rate determining.
E) None of the above could be correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys
Q59: At a particular temperature,N<sub>2</sub>O<sub>5</sub> decomposes according
Q60: The following questions refer to the
Q61: The reaction A <span class="ql-formula"
Q62: The initial rate of production of
Q64: What is the numerical value of the
Q65: For the second-order reaction NO(g)+ O<sub>3</sub>(g)
Q66: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="For the
Q67: The rate is constant over time.
Q68: Consider the following rate law: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"