For the Second-Order Reaction NO(g)+ O3(g) NO2(g)+ O2(g),the Rate Constant Has Been Measured to Be 1
Multiple Choice
For the second-order reaction NO(g) + O3(g) NO2(g) + O2(g) ,the rate constant has been measured to be 1.08 107 M-1 s-1 at 298 K and the activation energy has been measured to be 11.4 kJ/mol over the temperature range 195 K to 304 K.What is the rate constant at 233 K? (R = 8.3145 J K-1 mol-1)
A) 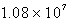 M-1 s-1
M-1 s-1
B) 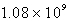 M-1 s-1
M-1 s-1
C) 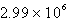 M-1 s-1
M-1 s-1
D) 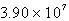 M-1 s-1
M-1 s-1
E) 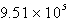 M-1 s-1
M-1 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q60: The following questions refer to the
Q61: The reaction A <span class="ql-formula"
Q62: The initial rate of production of
Q63: Two mechanisms are proposed: I. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q64: What is the numerical value of the
Q66: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="For the
Q67: The rate is constant over time.
Q68: Consider the following rate law: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q69: The elementary chemical reaction O +
Q70: The rate constant k is dependent on<br>I.the