Multiple Choice
Which orbital has the lowest energy in a multielectron atom? The quantum numbers are given as (n, 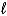 , ml) .
, ml) .
A) (3, 2, 1)
B) (5, 0, 0)
C) (4, 1, 1)
D) (4, 2, 1)
E) All are at equal energy.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following is not a
Q3: How many electrons can be in the
Q4: Which of the transitions in the hydrogen
Q5: Consider the radial distribution shown below for
Q6: Which of the following elements would you
Q7: Which listing has the orbitals in order
Q8: Which of the following sources produces the
Q9: If each of the following metals is
Q10: What is the orbital designation for an
Q11: How much energy is required to ionize