Multiple Choice
Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the smallest electrostatic potential energy (i.e., smallest in magnitude) ?
A) 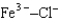
B) 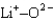
C) 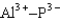
D) 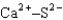
E) 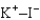
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q104: Use the following information to determine the
Q105: Internal energy is defined as _<br>A)the total
Q106: Predict the temperature change produced by burning
Q107: You hold a 50 g sphere of
Q108: Which arrow in the following diagrams represents
Q110: In terms of the enthalpy of formation,
Q111: What is the heat capacity (C<sub>p</sub>) of
Q112: Heat is best defined as _<br>A)a substance
Q113: Fuel value is _<br>A)the cost of energy
Q114: Which of the following substances will release