Multiple Choice
In terms of the enthalpy of formation, which of the following compounds is most stable relative to its elements under standard conditions?
A) PbBr2(s) , 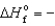 277.4 kJ/mol
277.4 kJ/mol
B) O3(g) , 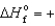 142.3 kJ/mol
142.3 kJ/mol
C) SO2(g) , 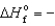 269.9 kJ/mol
269.9 kJ/mol
D) H2Se(g) , 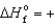 29.7 kJ/mol
29.7 kJ/mol
E) ICl(g) , 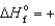 17.8 kJ/mol
17.8 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q105: Internal energy is defined as _<br>A)the total
Q106: Predict the temperature change produced by burning
Q107: You hold a 50 g sphere of
Q108: Which arrow in the following diagrams represents
Q109: Assuming that the distances between the two
Q111: What is the heat capacity (C<sub>p</sub>) of
Q112: Heat is best defined as _<br>A)a substance
Q113: Fuel value is _<br>A)the cost of energy
Q114: Which of the following substances will release
Q115: You have a summer job in a