Multiple Choice
What is the heat capacity (Cp) of a 7.5 g piece of tin if its temperature changes by 12.3C when it is supplied with 20 J from a Bunsen burner?
A) 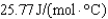
B) 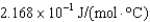
C) 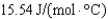
D) 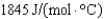
E) 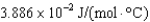
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: Predict the temperature change produced by burning
Q107: You hold a 50 g sphere of
Q108: Which arrow in the following diagrams represents
Q109: Assuming that the distances between the two
Q110: In terms of the enthalpy of formation,
Q112: Heat is best defined as _<br>A)a substance
Q113: Fuel value is _<br>A)the cost of energy
Q114: Which of the following substances will release
Q115: You have a summer job in a
Q116: Which of the following bar charts shows