Multiple Choice
You have a summer job in a lead foundry. Your task is to identify how energy efficiency can be improved. You therefore need to know the minimum amount of energy it takes to raise 1 pound of lead (454 g) from room temperature (25 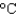 ) to its melting point (327
) to its melting point (327 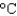 ) and then melt it. The specific heat capacity of lead is 0.159 J/(g
) and then melt it. The specific heat capacity of lead is 0.159 J/(g 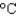 ) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
A) 3.39 MJ
B) 21.9 kJ
C) 21.0 kJ
D) 33.0 kJ
E) 11.0 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q110: In terms of the enthalpy of formation,
Q111: What is the heat capacity (C<sub>p</sub>) of
Q112: Heat is best defined as _<br>A)a substance
Q113: Fuel value is _<br>A)the cost of energy
Q114: Which of the following substances will release
Q116: Which of the following bar charts shows
Q117: Which one of the following statements is
Q118: In an experiment, 10.0 g of ice
Q119: When a 13.0 g sample of NaOH(s)
Q120: The cooling system in an automobile holds