Multiple Choice
In an experiment, 10.0 g of ice at 20C is converted into steam with a temperature of 110 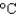 . How much energy is required for this process?
. How much energy is required for this process? 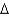 vap 2,260 J/g;
vap 2,260 J/g; 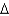 Hfus 334 J/g; cs(ice) 2.06 J/(g
Hfus 334 J/g; cs(ice) 2.06 J/(g 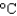 ) ; cs(water) 4.18 J/(g
) ; cs(water) 4.18 J/(g 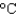 ) ; cs(steam) 1.99 J/(g
) ; cs(steam) 1.99 J/(g  ) )
) )
A) 30.7 kJ
B) 26.8 kJ
C) 34.9 kJ
D) 30.3 kJ
E) 38.7 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q113: Fuel value is _<br>A)the cost of energy
Q114: Which of the following substances will release
Q115: You have a summer job in a
Q116: Which of the following bar charts shows
Q117: Which one of the following statements is
Q119: When a 13.0 g sample of NaOH(s)
Q120: The cooling system in an automobile holds
Q121: Indicate which of the following is not
Q122: Assuming that the charge of the ions
Q123: Use the following information to determine the