Multiple Choice
Assuming that the charge of the ions remain constant in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i.e., largest in magnitude) ? 
A) 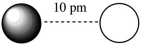
B) 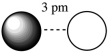
C) 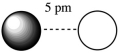
D) 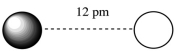
E) 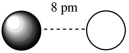
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q117: Which one of the following statements is
Q118: In an experiment, 10.0 g of ice
Q119: When a 13.0 g sample of NaOH(s)
Q120: The cooling system in an automobile holds
Q121: Indicate which of the following is not
Q123: Use the following information to determine the
Q124: In terms of the enthalpy of formation,
Q125: A 150 g piece of iron (C<sub>P</sub>
Q126: Using the following data for water, determine
Q127: At a certain elevation, the boiling point