Short Answer
Determine the enthalpy for the following reaction, given 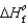 (NH3(g)) 46.1 kJ/mol,
(NH3(g)) 46.1 kJ/mol, 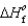 (NO(g))
(NO(g)) 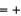 90.3 kJ/mol, and
90.3 kJ/mol, and 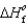 (H2O(g))
(H2O(g))  241.8 kJ/mol.
241.8 kJ/mol.
4NH3(g)  5O2(g)
5O2(g)  4NO(g)
4NO(g) 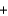 6H2O(g) Hrxn
6H2O(g) Hrxn  ? kJ
? kJ
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
F...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q14: The complete combustion of 2.500 g of
Q15: The heating curve for a substance is
Q16: A food sample was burned in a
Q17: Work requires _<br>A)a use of potential energy.<br>B)a
Q18: What is the change in internal energy
Q20: State Hess's law as it applies to
Q21: Use the following information to determine the
Q22: For which reaction below does the enthalpy
Q23: Benzoic acid is used to determine the
Q24: Describe the difference between the change in