Multiple Choice
Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions, and 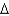
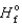 (NO2)
(NO2) 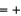 33.2 kJ/mol. 2NO(g)
33.2 kJ/mol. 2NO(g) 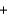 O2(g)
O2(g)  2NO2(g) Hrxn
2NO2(g) Hrxn  114.2 kJ
114.2 kJ
A) (90.3 kJ/mol)
B) (  90.3 kJ/mol)
90.3 kJ/mol)
C) (  147.4 kJ/mol)
147.4 kJ/mol)
D) (  180.6 kJ/mol)
180.6 kJ/mol)
E) (  73.7 kJ/mol)
73.7 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Q129: Which statement A-D about a system and
Q130: Use the following information to determine the
Q131: For which reaction below does the enthalpy
Q132: Which of the following fuels has the
Q133: Steam in a cylinder is compressed by
Q134: Using the data shown below for water,
Q135: To cool your 250 mL of coffee
Q137: Napthalene is often used to determine the
Q138: State the first law of thermodynamics. Describe
Q139: During a(n) _ process, energy is transferred