Multiple Choice
Use the following information to determine the enthalpy for the reaction shown below. S(s) O2(g) SO2(g) H ?
S(s) 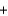
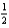 O2(g)
O2(g)  SO3(g)
SO3(g) 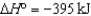 2SO2(g)
2SO2(g) 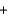 O2(g)
O2(g)  2SO3(g)
2SO3(g) 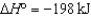
A) (593 kJ)
B) (593 kJ)
C) (296 kJ)
D) (296 kJ)
E) (989 kJ)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q125: A 150 g piece of iron (C<sub>P</sub>
Q126: Using the following data for water, determine
Q127: At a certain elevation, the boiling point
Q128: A 15 g piece of iron (C<sub>P</sub>
Q129: Which statement A-D about a system and
Q131: For which reaction below does the enthalpy
Q132: Which of the following fuels has the
Q133: Steam in a cylinder is compressed by
Q134: Using the data shown below for water,
Q135: To cool your 250 mL of coffee