Multiple Choice
In the reaction of carbon with oxygen,which substance is oxidized? The equation for the reaction is
C(s) + O2(g) 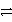
CO2(g) .
A) O2(g)
B) CO2(g)
C) No substance is oxidized in this reaction
D) C(s)
E) Both C(s) and O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In balancing the half-reaction<br>SO<sub>4</sub><sup>2</sup><sup>-</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg" alt="In
Q3: Identify the reduction half-reaction in the redox
Q5: What is the oxidizing agent in this
Q6: Which among the following are the parts
Q7: Consider the following beakers.In the beaker on
Q8: What is the reducing agent in the
Q10: What is the oxidation number of sulfur
Q11: Consider the following table of relative
Q12: What is the oxidation number of titanium
Q41: When zinc is plated to iron,the zinc