Exam 10: Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure
Exam 1: Structure and Bonding in Organic Molecules50 Questions
Exam 2: Structure and Reactivity: Acids and Bases,polar and Nonpolar Molecules39 Questions
Exam 3: Reactions of Alkanes: Bond-Dissociation Energies,radical Halogenation,and Relative Reactivity34 Questions
Exam 4: Cycloalkanes30 Questions
Exam 5: Stereoisomers44 Questions
Exam 6: Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic Substitution38 Questions
Exam 7: Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination25 Questions
Exam 8: Hydroxy of Functional Group: Alcohols: Properties,preparation,and Strategy of Synthesis39 Questions
Exam 9: Further Reactions of Alcohols and the Chemistry of Ethers46 Questions
Exam 10: Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure43 Questions
Exam 11: Alkenes: Infrared Spectroscopy and Mass Spectrometry47 Questions
Exam 12: Reactions to Alkenes44 Questions
Exam 13: Alkynes: the Carbon27 Questions
Exam 14: Delocalized Pi Systems: Investigation by Ultraviolet and Visible Spectroscopy Interlude34 Questions
Exam 15: Benzene and Aromaticity: Electrophilic Aromatic Substitution29 Questions
Exam 16: Electrophilic Attack on Derivatives of Benzene: Substituents Control Regioselectivity30 Questions
Exam 17: Aldehydes and Ketones: the Carbonyl Group32 Questions
Exam 18: Enols,enolates,and the Aldol Condensation: A,b-Unsaturated Aldehydes and Ketones31 Questions
Exam 19: Carboxylic Acids27 Questions
Exam 20: Carboxylic Acid Derivatives44 Questions
Exam 21: Amines and Their Derivatives: Functional Groups Containing Nitrogen20 Questions
Exam 22: Chemistry of the Benzene Substituents: Alkylbenzenes,phenols,and Benzenamines32 Questions
Exam 23: Ester Enolates and the Claisen Condensation: Synthesis of B-Dicarbonyl Compounds; Acyl Anion Equivalents29 Questions
Exam 24: Carbohydrates: Polyfunctional Compounds in Nature27 Questions
Exam 25: Heterocycles: Heteroatoms in Cyclic Organic Compounds23 Questions
Exam 26: Amino Acids,peptides,proteins,and Nucleic Acids: Nitrogen-Containing Polymers in Nature39 Questions
Select questions type
The CH2 group indicated would most likely appear as what in the proton NMR spectrum? 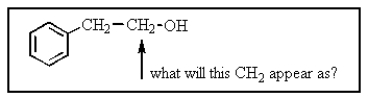
(Multiple Choice)
4.7/5  (31)
(31)
A chemist is evaluating a proton NMR spectrum taken on a 300 MHz NMR spectrometer.She wishes to determine the chemical shift in ppm of a singlet that appears at 1140 Hz from TMS.What is the chemical shift in ppm?
(Multiple Choice)
5.0/5  (35)
(35)
A compound of the formula C10H14 gave the 1H NMR spectrum shown below and exhibited three peaks in the 13C NMR spectrum.What compound might it be? 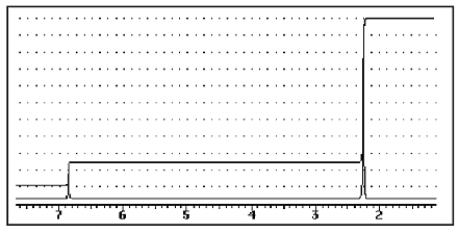
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is not true about the information from a proton NMR spectrum?
(Multiple Choice)
4.8/5  (44)
(44)
A bottle in a chemical stockroom was labeled simply "dichlorobenzene" without specifying which isomer was present.Capillary GC showed that it was a single pure compound,and the proton decoupled carbon NMR spectrum showed three peaks (not including solvent).You conclude that the bottle contained
(Multiple Choice)
4.8/5  (40)
(40)
The most downfield proton NMR signal (i.e.,signal most to the left in the spectrum)in the following molecule would be 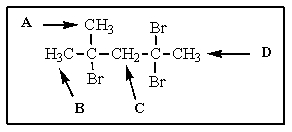
(Multiple Choice)
4.8/5  (37)
(37)
The following spectra data was most likely obtained from which compound? 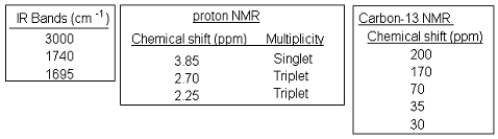
(Multiple Choice)
4.8/5  (39)
(39)
A very old bottle labeled only "chlorinated benzene" was found in the stockroom.Capillary GC showed (surprisingly)that the compound was pure,and a proton-decoupled 13C NMR spectrum showed only two peaks.Which of the following compounds was in the bottle?
(Multiple Choice)
4.9/5  (34)
(34)
Heating the 4-methylbenzenesulfonate ester of the isomer shown below in 2,2,2-trifluoroethanol (a highly ionizing solvent of low nucleophilicity)leads two products with the molecular formula C10H18.The major product displays 9 different signals in its 13C NMR spectrum.Two of them occur at relatively low field,about = 120 and 145 ppm,respectively.The 1H NMR spectrum exhibits a multiplet near = 5 ppm (1 H); all other signals are upfield of = 3 ppm.Identify the compound. 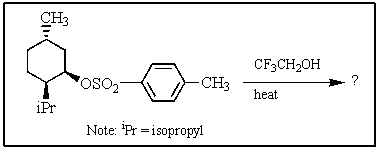
(Multiple Choice)
4.7/5  (40)
(40)
Which compound most likely exhibits the following proton NMR? 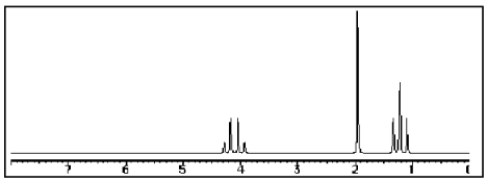
(Multiple Choice)
4.9/5  (31)
(31)
The type of electromagnetic energy required to cause nuclear magnetic resonance is
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following structures of formula C8H8Br2 would give the NMR spectrum shown below? 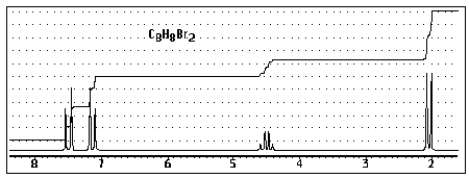
(Multiple Choice)
4.9/5  (38)
(38)
What structure would be consistent with the following NMR spectrum and data? 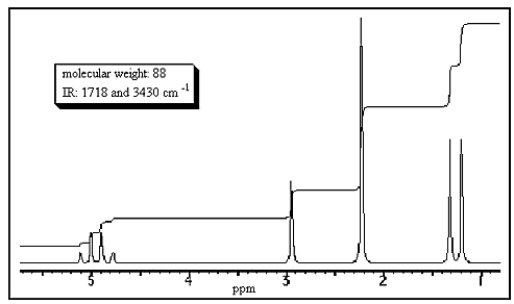
(Multiple Choice)
4.7/5  (38)
(38)
Describe the splitting that would be observed by Ha in the proton NMR spectrum,assuming Ha is coupled to all its neighboring protons in an equivalent manner. 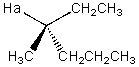
(Multiple Choice)
4.8/5  (36)
(36)
How many magnetically different types of hydrogens and carbons are in the following compound? 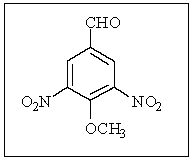
(Multiple Choice)
4.8/5  (43)
(43)
Which compound C9H11Br would give the proton NMR spectrum shown below? 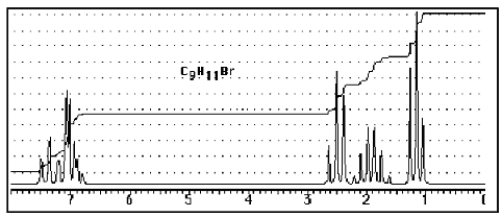
(Multiple Choice)
4.8/5  (39)
(39)
The molecular formulas and 13C NMR data (in ppm)are given below.The splitting pattern of each signal,taken from the non-decoupled spectrum is given in parentheses.Deduce the correct structure: 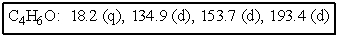
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following nuclei are incapable of magnetic resonance?
(Multiple Choice)
4.9/5  (38)
(38)
How many different signals will be present in the proton NMR for ethylpropanoate? (Do not count TMS as one of the signals!) 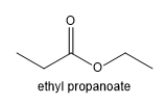
(Multiple Choice)
4.9/5  (43)
(43)
Showing 21 - 40 of 43
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)