Exam 6: An Overview of Organic Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds; Acids and Bases41 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry29 Questions
Exam 5: Stereochemistry at Tetrahedral Centers40 Questions
Exam 6: An Overview of Organic Reactions39 Questions
Exam 7: Alkenes and Alkynes36 Questions
Exam 8: Reactions of Alkenes and Alkynes38 Questions
Exam 9: Aromatic Compounds37 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy42 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy43 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy41 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations43 Questions
Exam 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides38 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions36 Questions
Exam 15: Carboxylic Acids and Nitriles36 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions46 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions46 Questions
Exam 18: Amines and Heterocycles36 Questions
Exam 19: Biomolecules: Amino Acids, Peptides, and Proteins52 Questions
Exam 20: Amino Acid Metabolism32 Questions
Exam 21: Biomolecules: Carbohydrates49 Questions
Exam 22: Carbohydrate Metabolism45 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism42 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism34 Questions
Exam 26: Orbitals and Organic Chemistry: Pericyclic Reactionse44 Questions
Exam 27: Synthetic Polymerse35 Questions
Select questions type
Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 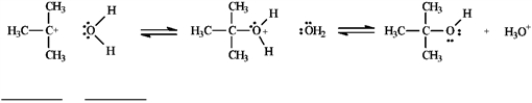 -Refer to instructions. Draw arrows on the structures above showing electron flow in steps two and three of this reaction.
-Refer to instructions. Draw arrows on the structures above showing electron flow in steps two and three of this reaction.
(Essay)
4.8/5  (37)
(37)
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 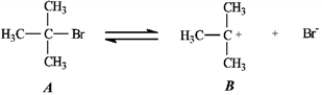 -Refer to instructions. Add curved arrows to indicate electron flow in the first step.
-Refer to instructions. Add curved arrows to indicate electron flow in the first step.
(Essay)
4.8/5  (39)
(39)
Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s).  -Refer to instructions. Label the nucleophile, Nu, and the electrophile, E+, in the blanks provided under the structures.
-Refer to instructions. Label the nucleophile, Nu, and the electrophile, E+, in the blanks provided under the structures.
(Essay)
4.7/5  (32)
(32)
Match each definition to one of the terms below.
-The energy needed by reactants to reach the transition state.
(Multiple Choice)
4.8/5  (44)
(44)
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
-If the yield for the following reaction is 76%, calculate Keq and predict the sign of ΔG°. 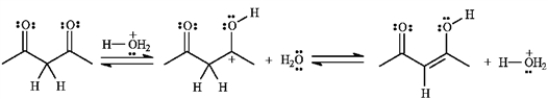
(Essay)
4.8/5  (28)
(28)
Use the reaction energy diagram below to answer the following question(s). 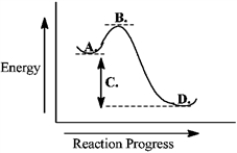 -The transition state is found at _____ on the diagram.
-The transition state is found at _____ on the diagram.
(Multiple Choice)
4.8/5  (32)
(32)
Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
-Classify and explain:
azide 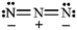
(Essay)
4.8/5  (42)
(42)
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
-Indicate flow: 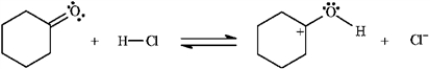
(Essay)
5.0/5  (27)
(27)
Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 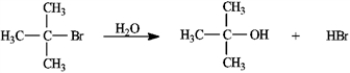 -Refer to instructions. This reaction is an example of:
-Refer to instructions. This reaction is an example of:
(Multiple Choice)
5.0/5  (35)
(35)
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s).  -In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps would be considered a chain termination step?
-In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps would be considered a chain termination step?
(Multiple Choice)
4.8/5  (33)
(33)
In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
-Label and indicate flow: 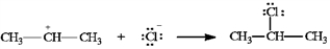
(Essay)
4.9/5  (37)
(37)
The reaction below is commonly used as a laboratory preparation of cyclohexene. Use this reaction to answer the following question(s). 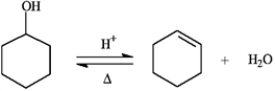 -Refer to instructions. If this reaction under a given set of conditions has a Keq value of 5.67, what percent conversion to the product would be expected?
-Refer to instructions. If this reaction under a given set of conditions has a Keq value of 5.67, what percent conversion to the product would be expected?
(Multiple Choice)
4.8/5  (35)
(35)
Match each definition to one of the terms below.
-A reaction where ΔG° is positive.
(Multiple Choice)
5.0/5  (32)
(32)
Which of the following correctly compares the two elements in terms of polarizability?
(Multiple Choice)
4.9/5  (40)
(40)
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
-The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step. 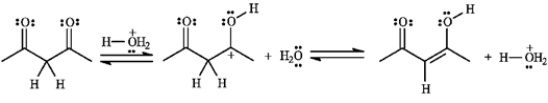
(Essay)
4.9/5  (45)
(45)
In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
-Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows. 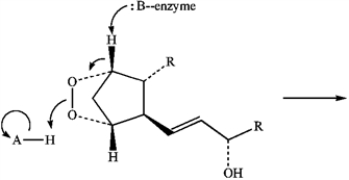
(Essay)
4.8/5  (38)
(38)
Identify the functional groups present in each compound below, and predict the direction of polarity in each.
-Identify and predict:
mustard gas Cl−CH2CH2−S−CH2CH2−Cl
(Essay)
4.8/5  (35)
(35)
Match each definition to one of the terms below.
-A reaction that involves a species with an unpaired electron.
(Multiple Choice)
4.9/5  (33)
(33)
Showing 21 - 39 of 39
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)