Exam 23: Carbonyl Condensation Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile. 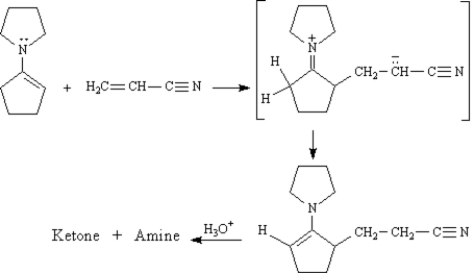 -Refer to Exhibit 23-10.Draw the structures of the Ketone + Amine products of this reaction.
-Refer to Exhibit 23-10.Draw the structures of the Ketone + Amine products of this reaction.
Free
(Essay)
5.0/5  (31)
(31)
Correct Answer:
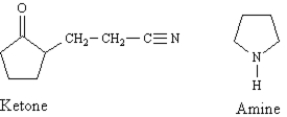
Which of the following would be classified as a Michael donor?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
Exhibit 23-2
Consider the reaction below to answer the following question(s): 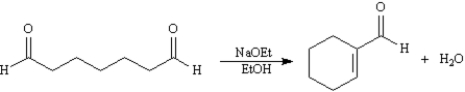 -Refer to Exhibit 23-2.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
-Refer to Exhibit 23-2.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
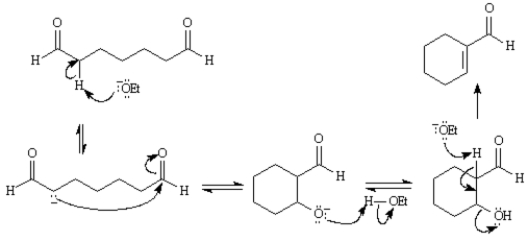
Exhibit 23-5
Consider the reaction below to answer the following question(s). 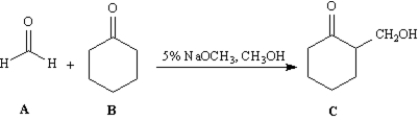 -Refer to Exhibit 23-5.This reaction is an example of:
-Refer to Exhibit 23-5.This reaction is an example of:
(Multiple Choice)
4.8/5  (27)
(27)
In the alkylation of cyclohexanone,better yields are obtained by first reacting cyclohexanone with an equivalent of lithium diisopropylamide in THF and then adding the alkyl halide,rather than mixing cyclohexanone,alkyl halide,and a catalytic amount of sodium ethoxide in ethanol.Explain this observation by pointing out what the problems with the second reaction conditions might be and how the first set of reaction conditions help alleviate the problems.
(Essay)
4.9/5  (28)
(28)
Show how you might use a Robinson annulation reaction to synthesize the following compound.Draw the structures of both reactants and the structure of the intermediate Michael addition product. 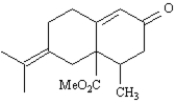
(Essay)
4.9/5  (39)
(39)
Draw the products of the Claisen condensation of the following substance.Atoms other than carbon and hydrogen are labeled. 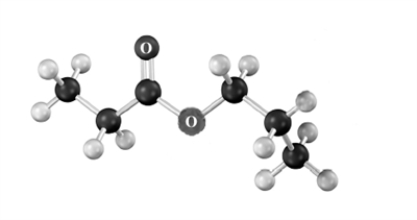
(Essay)
4.7/5  (38)
(38)
For which of the following will the aldol reaction go the furthest toward completion?
(Multiple Choice)
4.8/5  (36)
(36)
Exhibit 23-5
Consider the reaction below to answer the following question(s). 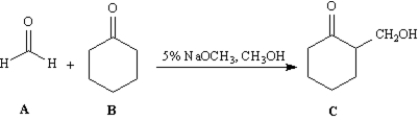 -Refer to Exhibit 23-5.Which carbonyl compound functions as the electrophile in this reaction?
-Refer to Exhibit 23-5.Which carbonyl compound functions as the electrophile in this reaction?
(Short Answer)
4.9/5  (43)
(43)
Draw the general mechanism of a carbonyl condensation reaction between two molecules of acetaldehyde.
(Essay)
4.9/5  (44)
(44)
The product of an aldol condensation reaction is generally favored if
(Multiple Choice)
4.9/5  (42)
(42)
Exhibit 23-7
Consider the reaction below to answer the following question(s):
Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 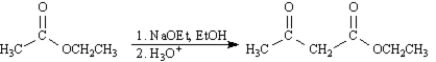 -Refer to Exhibit 23-7.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and draw all intermediate structures.
-Refer to Exhibit 23-7.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and draw all intermediate structures.
(Essay)
4.9/5  (40)
(40)
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.  -Refer to Exhibit 23-10.Show how you might use a Stork enamine reaction to prepare the following compound.
-Refer to Exhibit 23-10.Show how you might use a Stork enamine reaction to prepare the following compound. 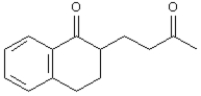
(Essay)
4.8/5  (34)
(34)
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.  -Refer to Exhibit 23-10.On the structures above,draw arrows indicating electron flow in each step of this reaction.
-Refer to Exhibit 23-10.On the structures above,draw arrows indicating electron flow in each step of this reaction.
(Essay)
4.8/5  (33)
(33)
Provide the indicated starting material and intermediate in the synthetic sequence below involving a Dieckmann cyclization,followed by a Robinson annulation. 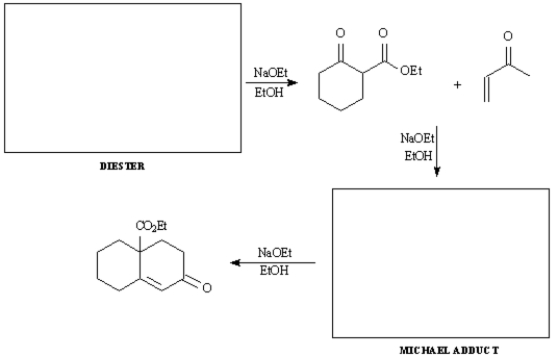
(Essay)
4.7/5  (37)
(37)
Exhibit 23-2
Consider the reaction below to answer the following question(s): 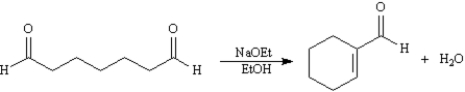 -Refer to Exhibit 23-2.This reaction is an example of:
-Refer to Exhibit 23-2.This reaction is an example of:
(Multiple Choice)
4.9/5  (40)
(40)
Consider the following molecular model.Atoms other than carbon and hydrogen are labeled. 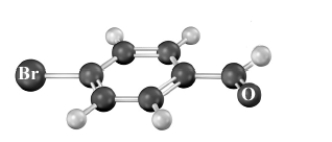 A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance.
A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance. 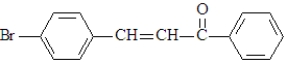 What is wrong with this synthesis?
What is wrong with this synthesis?
(Essay)
4.9/5  (37)
(37)
Which of the following when reacted with butanal would be the most likely to produce a single aldol product?
(Multiple Choice)
4.7/5  (27)
(27)
Showing 1 - 20 of 36
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)