Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
What is the correct structure for 2-hydroxyacetophenone?
Free
(Multiple Choice)
4.8/5  (47)
(47)
Correct Answer:
C
Exhibit 19-1
Consider the reaction below to answer the following question(s): 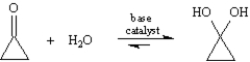 -Refer to Exhibit 19-1.The exact position of the equilibrium between ketones/aldehydes and their hydrates depends on the structure of the carbonyl compound.Although the equilibrium favors the carbonyl compound in most cases,cyclopropanone forms a stable hydrate.Explain this phenomenon based on the structures of cyclopropanone and its hydrate.
-Refer to Exhibit 19-1.The exact position of the equilibrium between ketones/aldehydes and their hydrates depends on the structure of the carbonyl compound.Although the equilibrium favors the carbonyl compound in most cases,cyclopropanone forms a stable hydrate.Explain this phenomenon based on the structures of cyclopropanone and its hydrate.
Free
(Essay)
4.8/5  (41)
(41)
Correct Answer:
The carbonyl carbon in cyclopropane is highly strained because the preferred bond angle for an sp2-hybridized carbon is 120° but the real angle is 60°.This angle strain is somewhat relieved when cyclopropanone is hydrated⎯the preferred bond angle for an sp3-hybridized carbon is about 109.5°.Going from sp2 to sp3 hybridization relieves angle strain by about 10°.Thus,the hydrate of cyclopropane is more stable than the carbonyl form,and the equilibrium lies to the right in this reaction.
Of the types of reactions that characterize carbonyl groups,how is the following reaction classified? 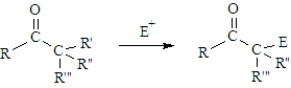
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
C
The following substance is heated in the presence of aqueous NaOH.Atoms other than carbon and hydrogen are labeled. 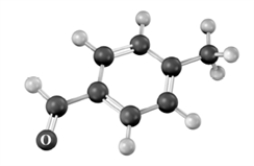 The product of the reaction is:
The product of the reaction is:
(Multiple Choice)
4.9/5  (36)
(36)
Which of the labeled peaks would allow the distinction of an aldehyde from a ketone based on this spectrum? 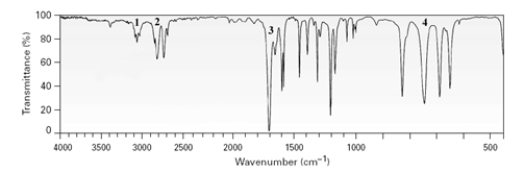
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following functional groups does not contain a carbonyl group?
(Multiple Choice)
4.8/5  (45)
(45)
Exhibit 19-10
Consider the data below to answer the following question(s).
C7H14O
IR: 1715 cm−1
MS: M+ at m/z = 114,α-cleavage fragment at m/z = 71,
no McLafferty rearrangement fragment
1H NMR: 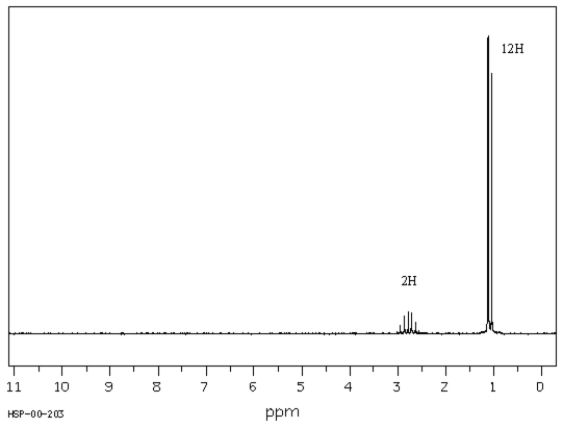 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS
-Refer to Exhibit 19-10.Propose a structure for this compound.
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS
-Refer to Exhibit 19-10.Propose a structure for this compound.
(Essay)
5.0/5  (36)
(36)
Exhibit 19-3
Consider the reaction below to answer the following question(s). 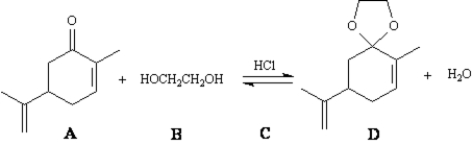 -Refer to Exhibit 19-3.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and show all intermediate structures.
-Refer to Exhibit 19-3.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and show all intermediate structures.
(Essay)
4.8/5  (34)
(34)
Exhibit 19-2
Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.Unfortunately,when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. 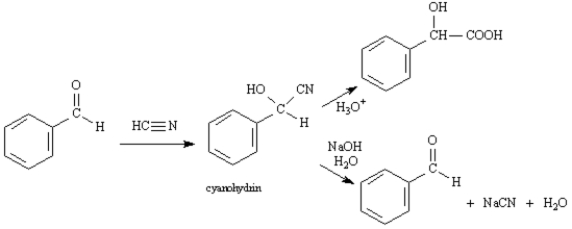 -Refer to Exhibit 19-2.Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.
-Refer to Exhibit 19-2.Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.
(Essay)
4.7/5  (34)
(34)
Exhibit 19-2
Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.Unfortunately,when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated.  -Refer to Exhibit 19-2.Formulate a reasonable mechanism for the reaction of the cyanohydrin of benzaldeyhe,shown above,with aqueous NaOH.Show all intermediate structures and all electron flow with arrows.
-Refer to Exhibit 19-2.Formulate a reasonable mechanism for the reaction of the cyanohydrin of benzaldeyhe,shown above,with aqueous NaOH.Show all intermediate structures and all electron flow with arrows.
(Essay)
4.9/5  (31)
(31)
In the Wittig reaction,a phosphorus ylide adds to a ketone or aldehyde to yield an alkene.Write the complete stepwise mechanism for the Wittig reaction shown below.Show all intermediate structures and all electron flow with arrows. 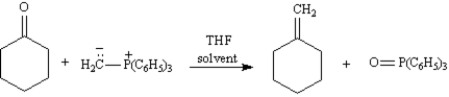
(Essay)
4.8/5  (45)
(45)
Exhibit 19-1
Consider the reaction below to answer the following question(s): 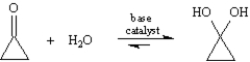 -Refer to Exhibit 19-1.Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base.Bases catalyze hydration by:
-Refer to Exhibit 19-1.Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base.Bases catalyze hydration by:
(Multiple Choice)
4.8/5  (42)
(42)
Exhibit 19-9
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114,α-cleavage fragment at m/z = 71,
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6H,triplet),1.59 δ (4H,multiplet),2.36 δ (4H,triplet)
-Refer to Exhibit 19-9.What functional group is indicated by the IR data?
(Essay)
4.7/5  (37)
(37)
Enamines formed from the cyclic secondary amine pyrrolidine are important intermediates in the synthesis of 1,5-diketones. 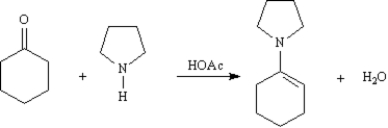 On the structures provided below,draw arrows showing electron flow for the reaction mechanism for the acetic acid-catalyzed formation of an enamine from cyclohexanone and pyrrolidine.
On the structures provided below,draw arrows showing electron flow for the reaction mechanism for the acetic acid-catalyzed formation of an enamine from cyclohexanone and pyrrolidine. 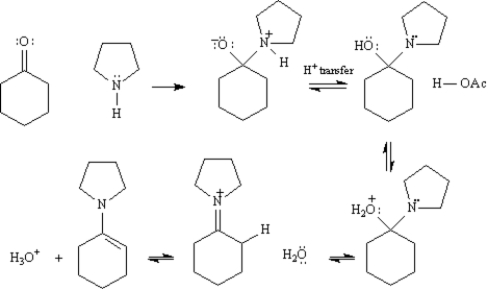
(Essay)
4.8/5  (37)
(37)
Showing 1 - 20 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)