Exam 5: Stereochemistry at Tetrahedral Centers
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Exhibit 5-7
A natural product having [α]D = +40.3° has been isolated and purified.
-Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice.
![Exhibit 5-7 A natural product having [α]<sub>D</sub> = +40.3° has been isolated and purified. -Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice. ](https://storage.examlex.com/TB4944/11eab917_151f_2441_99e6_dd784b5480ad_TB4944_00.jpg)
Free
(Essay)
4.8/5  (39)
(39)
Correct Answer:
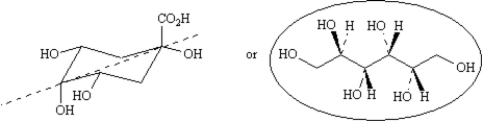
The information presented indicates that the natural product is optically active.To be optically active molecules must be chiral-that is,they must not have a plane of symmetry.The cyclic structure,although has chirality centers,has a plane of symmetry,indicated by the dashed line on the structure,and can,therefore,not be optically active.The circled structure has four chirality centers,and is not symmetric.We would expect it to be optically active.
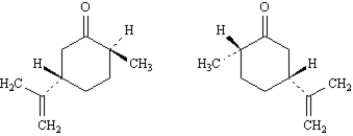
Exhibit 5-5
Label each pair of stereoisomers below as:
-_____ 
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
B
Exhibit 5-6
Refer to the structure below to answer the following question(s).
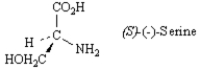 -Refer to Exhibit 5-6.Draw the enantiomer of (S)-(−)-serine in a wedge-dash projection.
-Refer to Exhibit 5-6.Draw the enantiomer of (S)-(−)-serine in a wedge-dash projection.
Free
(Essay)
4.9/5  (40)
(40)
Correct Answer:
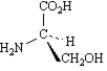
A white solid known to be enantiomerically pure is dissolved in water.This solution produced an observed optical rotation of +45.6°.The solution was allowed to stand and the optical rotation was measured repeatedly producing the data shown in the table.
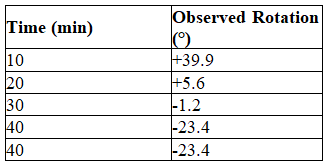 Explain the pattern of observed rotation.
Explain the pattern of observed rotation.
(Essay)
4.8/5  (41)
(41)
In muscles during strenuous exercise,under anaerobic conditions lactic acid builds up due to the following reaction. 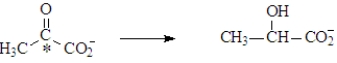 The carbon atom indicated by the asterisk is
The carbon atom indicated by the asterisk is
(Multiple Choice)
4.8/5  (25)
(25)
For which of the following generic substances is it not possible to isolate the enantiomers? (R1,R2 and R3 represent different groups. )
(Multiple Choice)
4.8/5  (40)
(40)
Exhibit 5-5
Label each pair of stereoisomers below as:
-_____ 
(Multiple Choice)
4.9/5  (35)
(35)
_____ is an sp3-hybridized atom that can become a chirality center by changing one of its attached groups.
(Multiple Choice)
4.8/5  (34)
(34)
Exhibit 5-5
Label each pair of stereoisomers below as:
-_____ 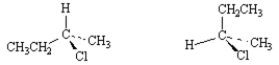
(Multiple Choice)
4.8/5  (49)
(49)
____ are organic molecules which rotate the plane of polarization of plane-polarized light.
(Multiple Choice)
4.7/5  (40)
(40)
_____ is the reason for "handedness" in molecules;the property of an object that causes it to be nonsuperimposable on its mirror image.
(Multiple Choice)
4.9/5  (38)
(38)
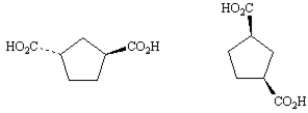
Exhibit 5-5
Label each pair of stereoisomers below as:
-_____ 
(Multiple Choice)
4.9/5  (44)
(44)
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.
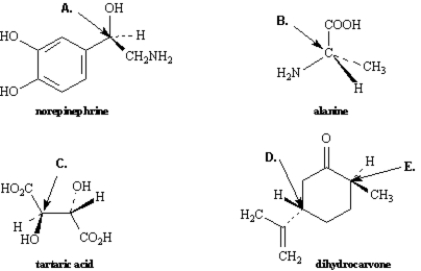 -Refer to Exhibit 5-3.The configuration of this carbon atom (E) is _____.
-Refer to Exhibit 5-3.The configuration of this carbon atom (E) is _____.
(Short Answer)
4.9/5  (34)
(34)
_____ are molecules which contain a plane of symmetry and chirality centers,but are achiral.
(Multiple Choice)
4.9/5  (42)
(42)
Exhibit 5-4
Consider the structure of streptimidone to answer the following question(s).
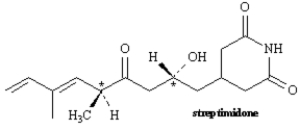 -Refer to Exhibit 5-4.Assign R or S configuration to each chirality center indicated in streptimidone.
-Refer to Exhibit 5-4.Assign R or S configuration to each chirality center indicated in streptimidone.
(Essay)
4.8/5  (47)
(47)
Exhibit 5-4
Consider the structure of streptimidone to answer the following question(s).
 -Refer to Exhibit 5-4.Will streptimidone have a meso stereoisomer? Explain your answer.
-Refer to Exhibit 5-4.Will streptimidone have a meso stereoisomer? Explain your answer.
(Essay)
4.7/5  (36)
(36)
Explain why a work glove is chiral but disposable surgical gloves are not.
(Essay)
4.8/5  (45)
(45)
Which of the following would be has the highest priority according to the sequence rules?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following arranges the groups in order of decreasing priority according to the sequence rules?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 1 - 20 of 43
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)