Exam 7: Alkenes: Structure and Reactivity
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Draw the mechanism of the electrophilic addition of HCl to 2-propene.
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
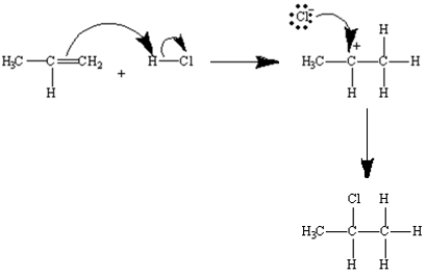
Exhibit 7-4
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
-Name: 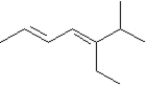
Free
(Short Answer)
4.8/5  (39)
(39)
Correct Answer:
(2E,4E)-5-ethyl-6-methyl-2,4-heptadiene
Identify the isoprene units in limonene,an essential oil found in oil of lemon and orange. 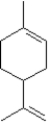
Free
(Essay)
4.7/5  (36)
(36)
Correct Answer:
There are two isoprene units.Bolded in the structure below. 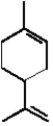
Cyclohexanol (-OH bonded to cyclohexane) produces cyclohexene when heated to 70 °C in the presence of a sulfuric acid catalyst.Write the equation for the reaction showing only the reactant of interest and the conditions of the reaction
(Essay)
4.7/5  (34)
(34)
Examine the following reaction. 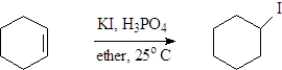 Which of the following is not indicated by this representation?
Which of the following is not indicated by this representation?
(Multiple Choice)
5.0/5  (46)
(46)
Exhibit 7-2
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
-Refer to Exhibit 7-2.Calculate the degree of unsaturation for Dieldrin.Show calculations for credit.
(Essay)
4.7/5  (32)
(32)
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.
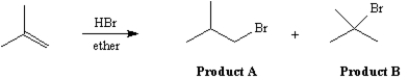 -Refer to Exhibit 7-13.Which product would be formed via a primary carbocation?
-Refer to Exhibit 7-13.Which product would be formed via a primary carbocation?
(Short Answer)
4.9/5  (38)
(38)
Exhibit 7-10
Consider the following reaction:
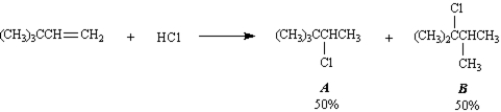 -Refer to Exhibit 7-10.Below are all the chemical structures and intermediates involved in this reaction.On the structures provided,show all electron flow using the arrow formalism for the complete stepwise mechanism for this reaction.
-Refer to Exhibit 7-10.Below are all the chemical structures and intermediates involved in this reaction.On the structures provided,show all electron flow using the arrow formalism for the complete stepwise mechanism for this reaction.
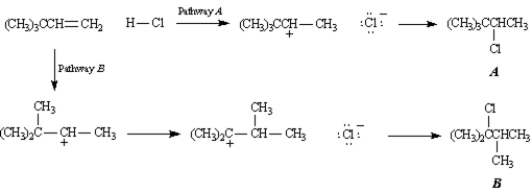
(Essay)
4.8/5  (41)
(41)
Which of the following does not characterize a more highly substituted carbocation?
(Multiple Choice)
4.7/5  (24)
(24)
Write the complete stepwise mechanism for the following reaction.Show all intermediate structures and all electron flow with arrows. 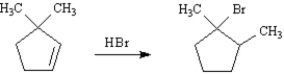
(Essay)
4.8/5  (47)
(47)
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.
 -Refer to Exhibit 7-13.Which product would be formed by a carbocation experiencing the greatest degree of hyperconjugative stabilization?
-Refer to Exhibit 7-13.Which product would be formed by a carbocation experiencing the greatest degree of hyperconjugative stabilization?
(Short Answer)
4.8/5  (43)
(43)
Exhibit 7-2
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
-Refer to Exhibit 7-2.How many double bonds does dieldrin have?
(Essay)
4.9/5  (32)
(32)
Rank the carbocations below in order of increasing stability (least stable = 1;most stable = 3).Place the number corresponding to the carbocation's relative stability in the blank below the structure. 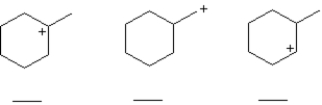
(Short Answer)
4.9/5  (40)
(40)
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.
 -Refer to Exhibit 7-13.According to Hammond's Postulate,which product would have a transition state structure most closely resembling the carbocation intermediate?
-Refer to Exhibit 7-13.According to Hammond's Postulate,which product would have a transition state structure most closely resembling the carbocation intermediate?
(Short Answer)
4.8/5  (39)
(39)
If the following compound was dissolved in ether and treated with HBr,which of the following describes the major product(s) of the reaction? 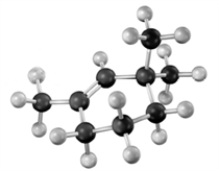
(Multiple Choice)
4.8/5  (24)
(24)
The following shows the connectivity of atoms only.Atoms other than carbon and hydrogen are labeled. 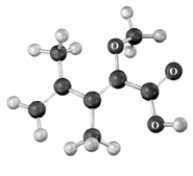 How many degrees of unsaturation are present in the compound?
How many degrees of unsaturation are present in the compound?
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following molecular model. 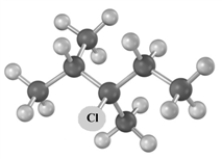 I Which of the following could be used to synthesize this compound?
I Which of the following could be used to synthesize this compound?
(Multiple Choice)
4.8/5  (38)
(38)
Exhibit 7-9
Consider the following reaction:
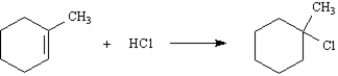 -Draw a qualitative reaction energy diagram for this reaction.Label the positions of all reactants,intermediates and products.
-Draw a qualitative reaction energy diagram for this reaction.Label the positions of all reactants,intermediates and products.
(Essay)
4.8/5  (32)
(32)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)