Exam 19: Work, heat, and the First Law of Thermodynamics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions49 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics II: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics38 Questions
Exam 42: Nuclear Physics64 Questions
Select questions type
What is the net power that a person loses through radiation if her surface area is 1.20 m2,if her emissivity is 0.895,if her skin temperature is 300 K,and if she is in a room that is at a temperature of 17°C? The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
If we use 67 W of power to heat 148 g of water,how long will it take to raise the temperature of the water from 15°C to 25°C? The specific heat of water is 4190 J/kg ∙ K.
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
A container of ideal gas has a movable frictionless piston.This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water.Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A,C
During an isothermal process,5.0 J of heat is removed from an ideal gas.How much work does the gas do during this process?
(Multiple Choice)
4.9/5  (36)
(36)
A chunk of ice (T = -20°C)is added to a thermally insulated container of cold water (T = 0°C).What happens in the container?
(Multiple Choice)
4.8/5  (37)
(37)
Two experimental runs are performed to determine the calorimetric properties of an alcohol that has a melting point of -10°C.In the first run,a 200-g cube of frozen alcohol,at the melting point,is added to 300 g of water at 20°C in a styrofoam container.When thermal equilibrium is reached,the alcohol-water solution is at a temperature of 5.0°C.In the second run,an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C.The specific heat of water is 4190 J/kg ∙ K.Assume that no heat is exchanged with the styrofoam container and with the surroundings.The heat of fusion of the alcohol is closest to
(Multiple Choice)
4.8/5  (35)
(35)
An ideal gas with γ = 1.67 is initially at 0°C in a volume of 10.0 L at a pressure of 1.00 atm.It is then expanded adiabatically to a volume of 10.4 L.What is the final temperature of the gas?
(Multiple Choice)
4.7/5  (35)
(35)
A person makes ice tea by adding ice to 1.8 kg of hot tea,initially at 80°C.How many kilograms of ice,initially at 0.00°C,are required to bring the mixture to 10°C? The heat of fusion of ice is 334 kJ/kg,and we can assume that tea has essentially the same thermal properties as water,so its specific heat is 4190 J/(kg ∙ K).
(Multiple Choice)
4.8/5  (36)
(36)
An adiabatic compression is performed on an ideal gas.The final pressure is equal to 0.560 times the initial pressure and the final volume equals 1.50 times the initial volume.What is the adiabatic constant for the gas?
(Multiple Choice)
4.8/5  (26)
(26)
A solid concrete wall 4.0 m by 2.4 m and 30 cm thick,with a thermal conductivity of 1.3 W/(m ∙ K),separates a basement at 18°C from the ground outside at 6°C.Under steady state conditions,how much heat flows through the wall in one hour?
(Multiple Choice)
4.9/5  (33)
(33)
If 2.0 g of water at 0.00°C is to be vaporized,how much heat must be added to it? The specific heat of water is 1.0 cal/g ∙ K,its heat of fusion is 80 cal/g,and its heat of vaporization is 539 cal/g.
(Multiple Choice)
4.8/5  (32)
(32)
A cylinder contains 24.0 moles of an ideal gas at a temperature of 300 K.The gas is compressed at constant pressure until the final volume equals 0.63 times the initial volume.The molar heat capacity at constant volume of the gas is 24.0 J/mol · K and the ideal gas constant is R = 8.314 J/mol ∙ K.The change in the internal (thermal)energy of the gas is closest to
(Multiple Choice)
4.8/5  (38)
(38)
Two experimental runs are performed to determine the calorimetric properties of an alcohol that has a melting point of -10°C.In the first run,a 200-g cube of frozen alcohol,at the melting point,is added to 300 g of water at 20°C in a styrofoam container.When thermal equilibrium is reached,the alcohol-water solution is at a temperature of 5.0°C.In the second run,an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C.The specific heat of water is 4190 J/kg ∙ K.Assume that no heat is exchanged with the styrofoam container and with the surroundings.The specific heat of the alcohol is closest to
(Multiple Choice)
4.8/5  (40)
(40)
The temperature of an ideal gas in a sealed 0.40 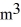 container is reduced from 400 K to
container is reduced from 400 K to 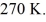 The final pressure of the gas is
The final pressure of the gas is 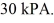 The molar heat capacity at constant volume of the gas is
The molar heat capacity at constant volume of the gas is 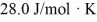 .The work done by the gas is closest to
.The work done by the gas is closest to
(Multiple Choice)
4.8/5  (36)
(36)
A cube at 100.0°C radiates heat at a rate of 80.0 J/s.If the length of each side is cut in half,the rate at which it will now radiate is closest to
(Multiple Choice)
4.9/5  (38)
(38)
The filament in a light bulb has a diameter of 0.050 mm and an emissivity of 1.0.The temperature of the filament is 3000°C.What should be the length of the filament so it will radiate 60 W of power? The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
(Multiple Choice)
4.8/5  (36)
(36)
A blacksmith is flattening a steel plate that measures 10 cm × 15 cm × 1 mm.He has heated the plate to 900 K.If the emissivity of the plate is 0.75,what is the total rate at which it radiates energy? The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.Ignore any heat it receives from the surroundings.
(Multiple Choice)
4.8/5  (30)
(30)
A heat conducting rod,1.40 m long,is made of an aluminum section that is 0.50 m long and a copper section that is 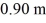 long.Both sections have cross-sectional areas of
long.Both sections have cross-sectional areas of 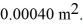 The aluminum end and the copper end are maintained at temperatures of
The aluminum end and the copper end are maintained at temperatures of 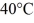 and
and 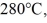 respectively.The thermal conductivity of aluminum is 205 W/m ∙ K of copper is 385 W/m ∙ K.The rate at which heat is conducted in the rod is closest to
respectively.The thermal conductivity of aluminum is 205 W/m ∙ K of copper is 385 W/m ∙ K.The rate at which heat is conducted in the rod is closest to
(Multiple Choice)
4.9/5  (25)
(25)
Showing 1 - 20 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)