Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction, 98.8 kJ of energy is absorbed.
Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 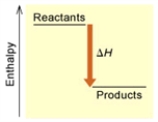 (A)
(A) 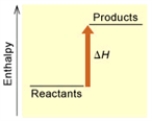 (B)
Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above
Represents this reaction?
(B)
Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above
Represents this reaction?
(Multiple Choice)
4.7/5  (33)
(33)
Determine the heat of evaporation of carbon disulfide, CS2( ) ? CS2(g)
Given the enthalpies of reaction below.
C(s) + 2 S(s) ? CS2( )
?rH° = +89.4 kJ/mol-rxn
C(s) + 2 S(s) ? CS2(g)
?rH° = +116.7 kJ/mol-rxn
(Multiple Choice)
4.9/5  (41)
(41)
Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below. (()\rightarrow()+(\ell) =65.2/- (()+()\rightarrow()+(\ell) =-113.8/- ()+()\rightarrow() =-393.5/- 2()+()\rightarrow2() =-1270.2/-
(Multiple Choice)
4.7/5  (38)
(38)
Given the thermochemical equation
4AlCl3(s) + 3O2(g) ? 2Al2O3(s) + 6Cl2(g); ?rH
= -529 kJ/mol-rxn
Find ?rH
For the following reaction.
Al2O3(s) + Cl2(g) ? AlCl3(s) + O2(g)
(Multiple Choice)
4.8/5  (28)
(28)
CaO(s) reacts with water to form Ca(OH)2(aq). If 6.50 g CaO is combined with 99.70 g H2O in a coffee cup calorimeter, the temperature of the resulting solution increases from 21.7 °C to 43.1 °C. Calculate the enthalpy change for the reaction per mole of CaO. Assume that the specific heat capacity of the solution is 4.18 J/g⋅K.
(Multiple Choice)
4.8/5  (35)
(35)
Calculate the energy in the form of heat (in kJ) required to convert 325 grams of liquid water at 20.0 °C to steam at 115 °C. Assume that no energy in the form of heat is transferred to the environment. (Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: liquid water = 4.184 J/g⋅K, steam = 1.92 J/g⋅K)
(Multiple Choice)
4.7/5  (38)
(38)
What quantity, in moles, of hydrogen is consumed when 179.6 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g)?
H2(g) + O2(g) ? H2O(l); ?rH° = -285.8 kJ/mol-rxn
(Multiple Choice)
4.9/5  (44)
(44)
How much heat is liberated at constant pressure if 0.515 g of calcium carbonate reacts with 32.7 mL of 0.498 M hydrochloric acid?
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g); ΔrH° = -15.2 kJ/mol-rxn
(Multiple Choice)
4.9/5  (32)
(32)
A chemical reaction in a bomb calorimeter evolves 3.86 kJ of energy in the form of heat. If the temperature of the bomb calorimeter increases by 4.17 K, what is the heat capacity of the calorimeter?
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following has zero standard enthalpy of formation at 25 °C?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid zinc nitrate?
(Multiple Choice)
4.8/5  (32)
(32)
If 35.0 g H2O at 22.7 °C is combined with 65.0 g H2O at 87.5 °C, what is the final temperature of the mixture? The specific heat capacity of water is 4.184 J/g⋅K.
(Multiple Choice)
4.9/5  (40)
(40)
Using the following thermochemical data:
2(s)+6(g)\rightarrow2(s)+3(g) =-1929.4/ mol-rxn 2(s)+6(g)\rightarrow2(s)+3(g) =-1498.0/-
Calculate ?rH° for the following reaction:
SmF3(s) + 3HCl(g) ? SmCl3(s) + 3HF(g)
(Multiple Choice)
4.8/5  (37)
(37)
The heat of vaporization of benzene, C6H6, is 30.7 kJ/mol at its boiling point of 80.1 °C. How much energy in the form of heat is required to vaporize 102 g benzene at its boiling point?
(Multiple Choice)
4.8/5  (33)
(33)
What is the standard enthalpy of formation of
CaCO3(s)+ CaO(s) + CO2(g) ? CaCO3(s); ?H° = -179.4 kJ/mol-rxn
Substance (/ mol-rxn ) (s) -634.9 (g) -393.5
(Multiple Choice)
4.8/5  (32)
(32)
What is the minimum mass of ice at 0.0 °C that must be added to 1.00 kg of water to cool the water from 28.0 °C to 12.0 °C? (Heat of fusion = 333 J/g; specific heat capacities: ice = 2.06 J/g⋅K, liquid water = 4.184 J/g⋅K)
(Multiple Choice)
5.0/5  (37)
(37)
Calculate the energy in the form of heat (in kJ) required to change 76.9 g of liquid water at 25.2 °C to ice at -15.2 °C. Assume that no energy in the form of heat is transferred to the environment. (Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g⋅K, liquid water = 4.184 J/g⋅K)
(Multiple Choice)
4.7/5  (39)
(39)
According to the reaction given below, the standard enthalpy change for the combustion of 1 mole of propane is -2043.0 kJ.
C3H8(g) + 5 O2(g) ? 3 CO2(g) + 4 H2O(g)
Calculate ?fH° for propane based on the given standard molar enthalpies of formation.
molecule \Delta(/ mol-rxn ) () -393.5 () -241.8
(Multiple Choice)
4.9/5  (42)
(42)
If 50.0 g of benzene (C6H6) absorbs 2.71 kJ of energy in the form of heat at 25.0 °C, what is the final temperature of benzene? The specific heat capacity of benzene is 1.72 J/g ⋅ K.
(Multiple Choice)
4.8/5  (49)
(49)
Exactly 149.6 J will raise the temperature of 10.0 g of a metal from 25.0 °C to 60.0 °C. What is the specific heat capacity of the metal?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 69
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)