Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
Which of the following energy diagrams represents the electronic state of the ground state of 1,3-butadiene? 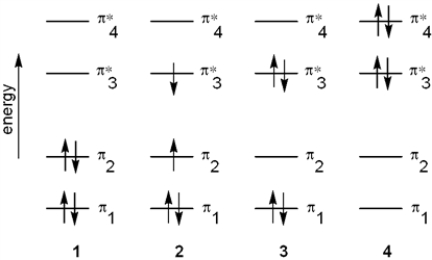
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H2/Pd 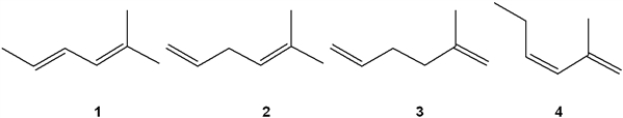
(Multiple Choice)
4.9/5  (35)
(35)
What is (are) the major organic product(s) formed in the following reaction? 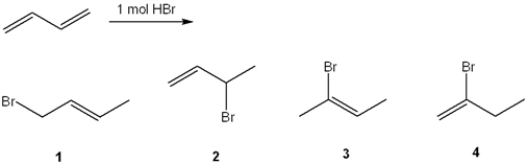
(Multiple Choice)
4.8/5  (40)
(40)
What are the units of ε in ultraviolet-visible spectroscopy?
(Multiple Choice)
4.9/5  (33)
(33)
Draw two resonance structures of the reactive intermediate that accounts for the formation of 1,2- and 1,4-addition products upon reaction of 1,3-butadiene with one equivalent of HBr.
(Essay)
4.8/5  (35)
(35)
Which of the following processes takes place upon absorption of ultraviolet-visible radiation?
(Multiple Choice)
4.9/5  (36)
(36)
Why carbonyl groups of aldehydes and ketones on conjugation with carbon-carbon double bonds show strong absorption in the ultraviolet spectrum?
(Essay)
4.7/5  (42)
(42)
Which of the following reaction coordinate diagrams explains the formation of different thermodynamic (T) and kinetic products from the same reactants (R)? 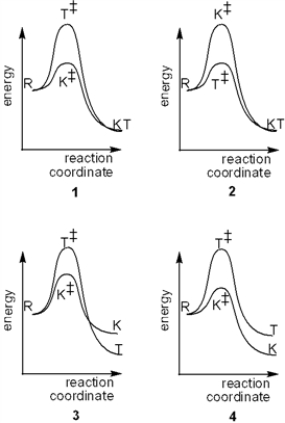
(Multiple Choice)
4.7/5  (37)
(37)
Sigmatropic shifts, cycloaddition reactions and anuulation reactions are all examples of pericyclic reactions.
(True/False)
4.9/5  (46)
(46)
What is the thermodynamic product obtained from the addition of 1 mole of bromine to 1,3-butadiene?
(Multiple Choice)
4.8/5  (43)
(43)
What is the systematic IUPAC name of the following compound? 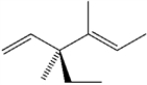
(Multiple Choice)
4.7/5  (38)
(38)
What is (are) the major organic product(s) formed in the following reaction? 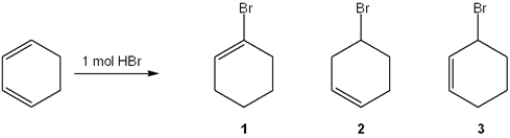
(Multiple Choice)
4.8/5  (36)
(36)
For a diene to undergo a Diels-Alder reaction it must be able to adopt an s-trans conformation.
(True/False)
4.9/5  (30)
(30)
Provide the structures of the constitutional isomers of the two major organic products obtained from the following reaction? 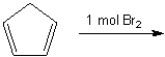
(Essay)
4.7/5  (37)
(37)
Consider the reaction below to answer the following question(s): 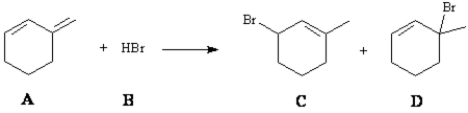 Enter the appropriate letter in the blank for each the following statements.
-The electrophile in this reaction is ____.
Enter the appropriate letter in the blank for each the following statements.
-The electrophile in this reaction is ____.
(Short Answer)
4.8/5  (41)
(41)
Consider the reaction below to answer the following question(s). 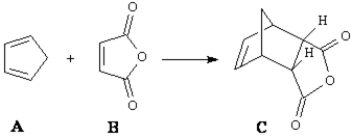 Fill in the blank in the question with the appropriate letter.
-The diene in the reaction is ____.
Fill in the blank in the question with the appropriate letter.
-The diene in the reaction is ____.
(Short Answer)
4.8/5  (33)
(33)
Which of the following terms describes the mechanism for the addition of Br2 to butadiene?
(Multiple Choice)
4.7/5  (30)
(30)
What are the two major organic products obtained from the following reaction? 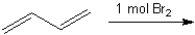
(Essay)
4.8/5  (32)
(32)
Which of 1,3-cyclohexadiene and 1,4-cyclohexadiene has the greatest heat of hydrogenation (i.e., the most exothermic reaction with H2 in the presence of a catalyst). Provide a brief explanation for your choice ?
(Essay)
4.9/5  (35)
(35)
Showing 41 - 60 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)