Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
The [2+2] cycloaddition reaction of ethylene to form cyclobutane does not occur because _____. ![The [2+2] cycloaddition reaction of ethylene to form cyclobutane does not occur because _____. ](https://storage.examlex.com/TB7078/11ead7c8_9d4c_0fbc_84b0_fbdf5d201147_TB7078_00.jpg)
(Multiple Choice)
4.8/5  (40)
(40)
Consider the following reaction. ![Consider the following reaction. This is an example of a sigmatropic reaction with a [3,3]-shift.](https://storage.examlex.com/TB7078/11ead7c8_9d49_9ea9_84b0_37fc0d07e320_TB7078_00.jpg) This is an example of a sigmatropic reaction with a [3,3]-shift.
This is an example of a sigmatropic reaction with a [3,3]-shift.
(True/False)
4.8/5  (35)
(35)
What is (are) the major organic product(s) formed in the following reaction? 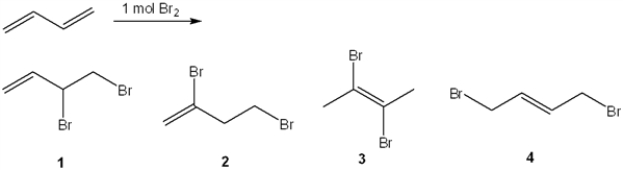
(Multiple Choice)
4.9/5  (36)
(36)
What is the reactive intermediate in the reaction of 1,3-butadiene with HBr resulting in 1,2-addition?
(Multiple Choice)
4.9/5  (39)
(39)
When an organic molecule is irradiated with ultraviolet radiation, the energy absorbed by the molecule corresponds to the amount necessary to excite electrons from one molecular orbital to another.
(True/False)
4.8/5  (34)
(34)
What is the kinetic product obtained from the addition of 1 mole of bromine to 1,3-butadiene?
(Multiple Choice)
4.9/5  (37)
(37)
Draw two resonance structures of the reactive intermediate that accounts for the formation of 1,2- and 1,4-addition products upon reaction of 1,3-butadiene with one equivalent of bromine.
(Essay)
4.9/5  (42)
(42)
Consider the reaction below to answer the following question(s): 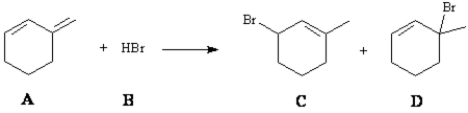 Enter the appropriate letter in the blank for each the following statements.
-The product that results from following mechanistic step is _____.
Enter the appropriate letter in the blank for each the following statements.
-The product that results from following mechanistic step is _____. 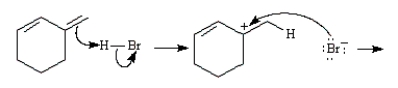
(Short Answer)
4.9/5  (40)
(40)
What is the major organic product obtained from the following reaction? 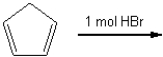
(Essay)
4.9/5  (35)
(35)
Which of the following has the highest value of λmax in the ultraviolet-visible spectrum? 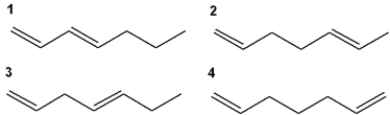
(Multiple Choice)
4.8/5  (35)
(35)
The molar absorptivity of ethylene in hexane is 15,000 L mol−1 cm−1. What is the maximum absorbance observed at a concentration of 1.5 x 10−4 mol L−1? (Path length = 1 cm)
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following relationships is not valid as applied to ultraviolet-visible spectroscopy?
(Multiple Choice)
4.8/5  (41)
(41)
What quantity is abbreviated as ε in ultraviolet-visible spectroscopy?
(Multiple Choice)
4.7/5  (31)
(31)
Consider the reaction below to answer the following question(s):  Enter the appropriate letter in the blank for each the following statements.
-The nucleophile in this reaction is ____.
Enter the appropriate letter in the blank for each the following statements.
-The nucleophile in this reaction is ____.
(Short Answer)
4.8/5  (30)
(30)
Which of the following energy diagrams represents the electronic state of butadiene after absorption of radiation? 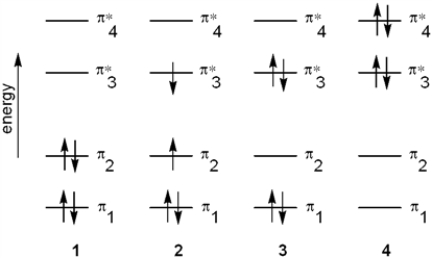
(Multiple Choice)
4.7/5  (42)
(42)
Showing 61 - 75 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)